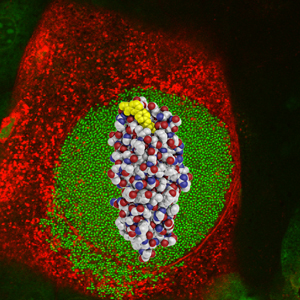
Scientists have found a way to block the “glue” that lets bacteria stick to the walls of the bladder and start a urinary tract infection. In this image, the glue (red, white and blue spheres) and the glue-blocker (yellow spheres) are overlaid on a micrograph of an infected bladder cell (red) filled with bacteria (green).
Bradley Ford
An experimental treatment for urinary tract infections has easily passed its first test in animals, alleviating weeks-long infections in mice in as little as six hours.
“This drug can block the spread of the bacteria that cause urinary tract infections far better than any other previously reported compound,” says senior author Scott J. Hultgren, PhD, the Helen L Stoever Professor of Molecular Microbiology at Washington University School of Medicine in St. Louis. “If it has similar effects in humans, the potential applications would be very exciting.”
The compound is a novel derivative of the natural sugar called mannose, making it unlikely to be toxic. Because the body normally directs excess sugars to the kidney for disposal in the urine, the new drug is naturally sent to exactly where it needs to be to treat infections of the urinary tract.
The results appear Nov. 16 in Science Translational Medicine.
Urinary tract infections mainly occur in women and are one of the most common infections, causing around $1.6 billion in medical expenses every year in the United States. Scientists believe 80 percent to 90 percent of these infections are caused by the bacterium Escherichia coli (E. coli). Half of all women will experience a urinary tract infection at some point in their lives, and additional recurrent infections will affect 20 percent to 40 percent of these women.
Doctors currently treat urinary tract infections with antibiotics. But about 10 percent to 30 percent of these infections are antibiotic resistant, and resistance is spreading rapidly.
Hultgren has shown that the bacteria that cause urinary tract infections create long hair-like fibers on their surfaces that are tipped with a protein that acts like glue. These glue-tipped fibers allow them to cling to receptors on the bladder wall instead of being flushed out by urine.
“Binding to host cells is the key event in most bacterial infections because it’s the step that has to be taken before the bacterium can infect the cell,” Hultgren says.
The glue also helps bacteria stick to each other in cooperative communities, increasing their resistance to drugs and the immune system.
James W. Janetka, PhD, research assistant professor of biochemistry and molecular biophysics, designed a group of investigational drugs based on information from Hultgren’s research. Scientists call these drugs mannosides because they are derivatives of mannose and block the bacterial glue’s ability to bind to mannose found on the surface of cells in the bladder. They designed and tested a number of these compounds, and chose one finalist from among the top six candidates.
When scientists fed the most promising drug to mice with chronic urinary tract infections, bacteria levels in their bladders declined much more quickly than in a second group of mice given antibiotics. The mannoside also prevented new infections.
“In other tests, when we gave infected mice the mannoside first, the bacteria became much more vulnerable to treatment with antibiotics,” Janetka says. “But we produced our most exciting results when we used the mannoside and the antibiotics simultaneously. This created a synergistic effect in the mice that could rapidly eliminate even infections by antibiotic-resistant bacterial strains.”
Hultgren says it should be much harder for bacteria to develop resistance to the mannoside.
“Antibiotics have to get into the bacterial cell to kill it,” Hultgren says. “This gives the bacteria a number of opportunities to resist: They can change their cell walls to make it harder for the antibiotic to get in, or they can destroy the antibiotic after it gets in or pump it back out of the cell. Mannosides bypass those resistance mechanisms because they don’t have to get into the cell.”
Hultgren and Janetka are continuing to develop more potent mannosides. They hope to begin human toxicity tests for the drugs late in 2012. If those tests are successful, clinical trials may follow.
According to Hultgren, the approach he and Janetka are using to develop the mannosides could be harnessed to treat infections in other parts of the body, such as ear or kidney infections.
“Cells in other parts of the body have different materials on their surfaces, and bacteria that cause infections in those areas will have proteins that are customized to stick there,” he says. “Blocking binding proteins could be helpful in fighting a wide variety of bacterial infections.”
Washington University holds a patent on the mannosides developed by Hultgren and Janetka. Their interests in these compounds are managed by the Office of Technology Management in accordance with the University’s policies on intellectual property.
Cusumano CK, Pinkner JS, Han Z, Greene SE, Ford BA, Crowley JR, Henderson JP, Janetka JW, Hultgren SJ. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Science Translational Medicine, Nov. 16, 2011.
Funding from the National Institutes of Allergy and Infectious Diseases, the National Institute of Diabetes, Digestive and Kidney Diseases, the American Recovery and Reinvestment Act, the Office of Research on Women’s Health and the Burroughs Wellcome Fund supported this research.
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked fourth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.