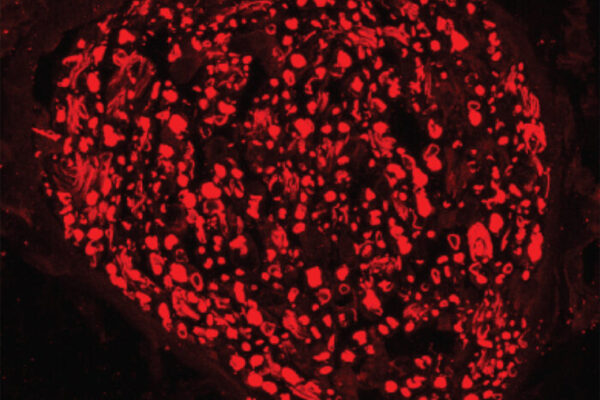
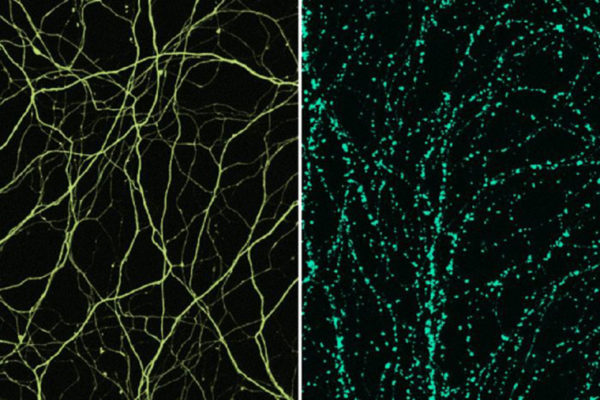
A protein required to regrow injured peripheral nerves has been identified by researchers at Washington University School of Medicine in St. Louis.
The finding, in mice, has implications for improving recovery after nerve injury in the extremities. It also opens new avenues of investigation toward triggering nerve regeneration in the central nervous system, notorious for its inability to heal.
Peripheral nerves provide the sense of touch and drive the muscles that move arms and legs, hands and feet. Unlike nerves of the central nervous system, peripheral nerves can regenerate after they are cut or crushed. But the mechanisms behind the regeneration are not well understood.
In the new study, published online June 20 in Neuron, the scientists show that a protein called dual leucine zipper kinase (DLK) regulates signals that tell the nerve cell it has been injured – often communicating over distances of several feet. The protein governs whether the neuron turns on its regeneration program.

“DLK is a key molecule linking an injury to the nerve’s response to that injury, allowing the nerve to regenerate,” says Aaron DiAntonio, MD, PhD, professor of developmental biology. “How does an injured nerve know that it is injured? How does it take that information and turn on a regenerative program and regrow connections? And why does only the peripheral nervous system respond this way, while the central nervous system does not? We think DLK is part of the answer.”The nerve cell body containing the nucleus or “brain” of a peripheral nerve resides in the spinal cord. During early development, these nerves send long, thin, branching wires, called axons, out to the tips of the fingers and toes. Once the axons reach their targets (a muscle, for example), they stop extending and remain mostly unchanged for the life of the organism. Unless they’re damaged.
If an axon is severed somewhere between the cell body in the spinal cord and the muscle, the piece of axon that is no longer connected to the cell body begins to disintegrate. Earlier work showed that DLK helps regulate this axonal degeneration. And in worms and flies, DLK also is known to govern the formation of an axon’s growth cone, the structure responsible for extending the tip of a growing axon whether after injury or during development.
The formation of the growth cone is an important part of the early, local response of a nerve to injury. But a later response, traveling over greater distances, proves vital for relaying the signals that activate genes promoting regeneration. This late response can happen hours or even days after injury.
But in mice, unlike worms and flies, DiAntonio and his colleagues found that DLK is not involved in an axon’s early response to injury. Even without DLK, the growth cone forms. But a lack of DLK means the nerve cell body, nestled in the spinal cord far from the injury, doesn’t get the message that it’s injured. Without the signals relaying the injury message, the cell body doesn’t turn on its regeneration program and the growth cone’s progress in extending the axon stalls.
In addition, it was shown many years ago that axons regrow faster after a second injury than axons injured only once. In other words, injury itself increases an axon’s ability to regenerate. Furthering this work, first author Jung Eun Shin, graduate research assistant, and her colleagues found that DLK is required to promote this accelerated growth.

“A neuron that has seen a previous injury now has a different regenerative program than one that has never been damaged,” Shin says. “We hope to be able to identify what is different between these two neurons — specifically what factors lead to the improved regeneration after a second injury. We have found that activated DLK is one such factor. We would like to activate DLK in a newly injured neuron to see if it has improved regeneration.”In addition to speeding peripheral nerve recovery, DiAntonio and Shin see possible implications in the central nervous system. It is known for example, that some of the important factors regulated and ramped up by DLK are not activated in the central nervous system.
“Since this sort of signaling doesn’t appear to happen in the central nervous system, it’s possible these nerves don’t ‘know’ when they are injured,” DiAntonio says. “It’s an exciting idea — but not at all proven — that activating DLK in the central nervous system could promote its regeneration.”
Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. Online June 20, 2012.
This work was supported by the National Institutes of Health (NIH) Neuroscience Blueprint Center Core Grant (P30 NS057105) to Washington University, the HOPE Center for Neurological Disorders, the European Molecular Biology Organization (EMBO) long-term fellowship, the Edward Mallinckrodt Jr. Foundation, and NIH grants NS060709, AG13730, NS070053 and NS065053.
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.