A noninvasive approach for assessing lung inflammation should accelerate efforts to develop drugs for inflammatory lung conditions like cystic fibrosis and pneumonia, scientists at Washington University School of Medicine in St. Louis report.
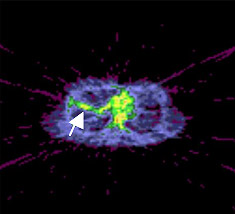
Researchers have used positron emission tomography (PET) scans to monitor artificially induced inflammation in the lungs of healthy volunteers. The new imaging process may help doctors monitor the conditions of patients with inflammatory lung diseases and should make it easier to test potential anti-inflammatory drugs in trials.
“Until now, when we wanted to assess whether a new drug decreased lung inflammation, the options for specifically measuring active inflammation were not pleasant,” says lead author Delphine Chen, M.D., chief resident in nuclear medicine at the medical school’s Mallinckrodt Institute of Radiology. “We could perform a bronchoscopy and gather samples directly from the breathing passages, or we could have patients inhale a saline solution and cough it back up.”
To make it possible to detect lung inflammation with PET, Chen and her colleagues employed an imaging technique commonly used to diagnose cancer and monitor its treatment. Scientists reported the results in a paper published online by The Journal of Applied Physiology.
Senior author Daniel P. Schuster, M.D., professor of medicine and of radiology, hopes the new imaging process will make it possible to give new drugs trial runs.
“Full-scale clinical trials are costly in terms of both time and dollars spent, and right now it’s very difficult to find intermediate steps that allow us to build confidence in a drug’s effectiveness before taking that plunge,” Schuster says.
With the new PET procedure, Schuster says, researchers developing anti-inflammatory drugs can test the drugs’ effects in less expensive trials involving smaller groups of healthy volunteers and patients.
“If the drug passes those tests, then you can say, okay, let’s see in a full-scale trial if the drug actually has an impact on some important patient-centered outcome like mortality or disease progression,” he says.
To create areas of limited lung inflammation in healthy volunteers, researchers used a technique originally developed by scientists at the National Institutes of Health. It involves the injection, via bronchoscope, of a small amount of endotoxin into a lung segment.

“Endotoxin is a purified bacterial substance that triggers inflammation,” Schuster says. “The technique we used keeps that inflammation compartmentalized to a small region in the lung, ensuring that the inflammation doesn’t become systemic.”
Another research group previously had shown that this artificial inflammation could be used to test potential drugs, but they followed the effects of the drugs via a second insertion of the bronchoscope into the volunteers’ tracheas.
Chen and her colleagues instead injected volunteers with fluorodeoxyglucose (FDG), a form of sugar readily detectable by PET, and continuously monitored the lungs for 60 minutes to see how much FDG appeared there.
Scientists already have completed a trial to test the new imaging procedure’s ability to detect inflammation in cystic fibrosis patients that will be published soon in The American Journal of Respiratory and Critical Care Medicine.
Chen DL, Rosenbluth DB, Mintun MA, Schuster DP. FDG-PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. The Journal of Applied Physiology, online publication.
Funding from the National Institutes of Health and the Cystic Fibrosis Foundation supported this research.
Washington University School of Medicine’s full-time and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked third in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.