Respiratory conditions that restrict breathing such as asthma and chronic obstructive pulmonary disease (COPD) are common killers worldwide. But no effective treatments exist to address the major cause of death in these conditions – excess mucus production.
“There is good evidence that what kills people with severe COPD or asthma is mucus obstructing the airway,” says Michael J. Holtzman, MD, the Selma and Herman Seldin Professor of Medicine at Washington University School of Medicine in St. Louis. “It’s a huge unmet medical problem and is only increasing in this country and throughout the world.”
Now, Holtzman and his colleagues have described the molecular pathway responsible for excess mucus in airway cells and have used that information to design a series of new drugs that inhibit that pathway.
Their study appears online Nov. 26 in the Journal of Clinical Investigation.
Chronic respiratory disease, especially COPD, is the third leading cause of death in the United States and worldwide. Smoking and exposure to pollution are major causes of these diseases. Related conditions that affect the respiratory airways, like asthma and bronchitis, are also among the most common causes of human disease in adults and children. The morbidity and mortality from these conditions is closely linked to excess mucus production that blocks the airways and prevents normal breathing. However, there are no effective treatments to address the overproduction of airway mucus.
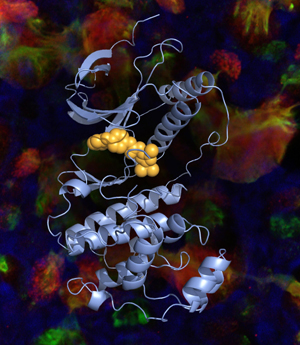
As part of the new research, the scientists discovered that a critical signaling molecule, CLCA1, has a special role in the mucus pathway. They showed that CLCA1 allows a protein known as IL-13 to turn on the major mucus gene in airway cells. The researchers also showed that CLCA1 needs help from an enzyme called MAPK13.
Although there were no existing drugs that acted against MAPK13, Holtzman says there were several that inhibit a similar enzyme known as MAPK14, which differs slightly in structure.

“We could take advantage of the MAPK14 inhibitors that were already known,” Holtzman says. “These drugs bind to a specific pocket in MAPK14 to block its activity. For MAPK13, that pocket itself has some obstructions making it more crowded and harder to access, so these older drugs can’t fit into the pocket to block activity.”
So Holtzman and his team built drugs with slimmer structures that could avoid the obstacles and better fit into the protein pocket of MAPK13.
“We sculpted the drugs so they’re better able to bind in the MAPK13 pocket,” Holtzman says. “And we showed that the new shape translates into more potent and effective blockade of MAPK13 activity. It’s drug discovery that takes advantage of biology and chemistry, and it takes a very special team to do that.”
Indeed, the results show that some of their newly designed MAPK13 inhibitors reduced mucus production in cultures of human airway cells by 100 fold.
Importantly, Holtzman says that this work had to be done in human cells because commonly used lab animals have different wiring for the mucus production circuit. For example, MAPK13 inhibitors were not effective in mice because other types of CLCA and MAPK proteins could continue to make excess mucus.
“We recognized that we had to work directly in human cells to figure out the control system,” Holtzman says. “We then looked at lungs from patients with very severe COPD who were at the last resort of lung transplantation. In these diseased lungs, we found too much mucus, too much CLCA1 and over-activated MAPK13. What we observed in isolated human cells translated to a devastating disease in real life.”
Beyond COPD and asthma, Holtzman also sees a possible role for MAPK13 inhibitors in related conditions with excess mucus production, like cystic fibrosis and even the common cold.
“The big killer is COPD,” Holtzman says. “But other extra-mucus conditions can also be life threatening. And we know from our studies that respiratory viral infections and allergies are potent activators of this same pathway for mucus production. Since the new inhibitors would be active in both the upper and lower airways, they could impact a wide set of respiratory illnesses.”
Editor’s Note: Holtzman and his colleagues worked with the Office of Technology Management to file a provisional patent on the new compounds developed in this study.
Journal of Clinical Investigation. Online Nov. 26, 2012.
This work was supported by grants from the National Institutes of Health (NIAID AADCRC U19-AI070489 and MRCE U54-AI05160, and NHLBI P01-HL29594, R01-HL073159, CADET P50-HL107183, and K08-HL083095) and the Martin Schaeffer Fund. It was further supported by the Flow Cytometry Core in the Siteman Cancer Center and the Cores in Pulmonary and Critical Care Medicine. These Cores include the Epithelial Cell Core supported by the Children’s Discovery Institute. Structural results were derived from work performed at Argonne National Laboratory (ANL) Structural Biology Center and the Advanced Light Source, Berkeley CA (ALS). ANL is operated by U. Chicago Argonne, LLC, for the U.S. DOE, Office Biological and Environmental Research (DE-AC02-06CH11357). ALS is supported by the Office of Basic Energy Sciences of the U.S. DOE (DE-AC02-05CH11231). Confocal microscopy was derived in part from work in the Microscopy and Digital Imaging Core of the University Research Center for Auditory and Vestibular Studies supported by NIH NICDD P30DC04665.
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.